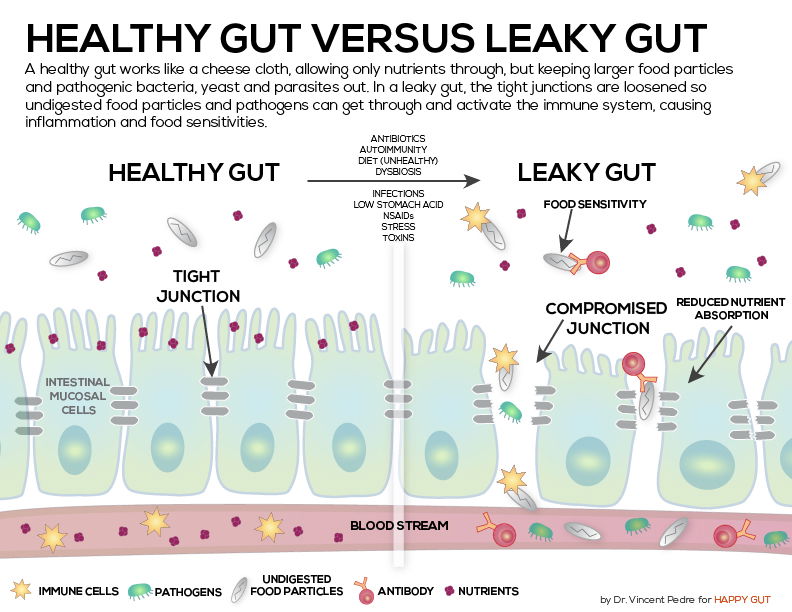
Food allergies were linked to increased disease activity in patients with multiple sclerosis (MS), researchers reported.
MS patients with food allergies had more relapses and a higher likelihood of gadolinium-enhancing lesions on MRI than patients with no known allergies, reported Tanuja Chitnis, MD, of Brigham and Women's Hospital in Boston, and colleagues in the Journal of Neurology, Neurosurgery & Psychiatry.
"Food allergies perturb the immune system in ways that seem to increase MS inflammatory activity," Chitnis told MedPage Today.
"Our results highlight a previously unknown relationship between food allergies and the incidence of inflammatory new lesions in multiple sclerosis," she added. "This finding could lead to new therapeutic strategies to curtail and prevent relapses in MS patients."
Other studies have examined the relationship between MS and allergies as well as the coexistence of other autoimmune diseases with conflicting results, noted Bianca Weinstock-Guttman, MD, of the University of Buffalo in New York, who was not involved with the research.
"Interestingly, a previous study on pediatric MS patients identified that food allergies that developed within the first 5 years of life were associated with a lower risk for relapses," Weinstock-Guttman told MedPage Today. "Immunological differences developed too early versus later-life food allergens between the pediatric to adult population may be an explanation for the discordant results."
In this study, a subset of 1,349 patients enrolled in the Comprehensive Longitudinal Investigation of Multiple Sclerosis at the Brigham and Women's Hospital (CLIMB) study completed a self-administered questionnaire about environmental, food, and drug allergies. All patients had an MS diagnosis according to 2010 McDonald criteria; patients with a known allergy to any disease-modifying therapy were excluded from the analysis.
The average age of patients was about 50, and mean disease duration was about 16 years. About 75% of patients had relapsing remitting multiple sclerosis (RRMS). More than half of the sample (753 patients) had an MRI scan with gadolinium within ±90 days of the clinic visit when the allergy questionnaire was completed.
In total, 922 MS patients reported any allergies. More men reported no allergies (35%) than any allergies (21%); other variables were comparable between the two groups. Expanded Disability Status Scale (EDSS) scores and Multiple Sclerosis Severity Scores (MSSS) were similar between patients with allergies and those without.
The researchers grouped patients into one of four groups: environmental allergies (n=586), food allergies (n=238), drug allergies (n=574), and no known allergies (n=427).
Multivariable analysis controlled for sex, age at symptom onset, race, disease category, and percentage of time on disease-modifying therapy. Having any allergies was associated with a 1.22 times higher rate for cumulative attacks compared with the no-allergy group (P=0.0204) in univariate analysis, but in adjusted analysis, this difference disappeared (P=0.1497).
When stratified by allergy, however, the food group had a 1.38 times higher rate for cumulative number of attacks than patients with no known allergies (P=0.0062). In adjusted analysis, this difference remained significant (1.27; P=0.0305). In contrast, the environmental and drug allergy groups did not show significant differences compared with the no-allergy group.
The food allergy group also showed more than twice the likelihood (OR 2.53, 95% CI 1.25-5.11; P=0.0096) of having gadolinium-enhancing lesions on MRI.
"The microbiome known to interfere with the systemic immune system probably is an important factor," observed Weinstock-Guttman.
"Further studies on microbiome and dietary interventions, especially in patients with specific food allergies, may provide helpful knowledge for better and more specific control," she said. "The influence of specific disease-modifying therapies that can affect disease outcome but also change the microbiome should be considered."
This analysis was based on a cross-sectional study; it cannot establish causality, Chitnis and colleagues noted. It was limited by self-reported allergies that were not confirmed by an allergy specialist. The questionnaire could not differentiate between true food allergy and other adverse reactions to food, they added. The study also had no information about patients' MS treatments.
The CLIMB study is supported by Merck Serono and the National MS Society Nancy Davis Center Without Walls.
The researchers reported relationships with Verily Life Sciences, Novartis, Merck Serono, Genentech, Biogen, Teva Pharmaceuticals, Sanofi, Tilos Therapeutics, Tiziana Life Sciences, IM Therapeutics, vTv Therapeutics, MedDay Pharmaceuticals, Bayer, and Celgene.